Acid-base indicators change colour in acidic or basic solutions. Neutralization between a strong acid HCl and a strong base NaOH is represented by H Cl- Na OH- Na Cl- H 2 O It is evident from the above equation that as NaOH solution is gradually added the H ion having high ionic conductance are replaced by Na having lower ionic conductance and hence the conductivity of the solution gradually decrease.
Phenolphthalein indicator changes color in the pH range of 83 to 100 and can be used to determine when the correct amount of base has been added.

. The acid-base indicator indicates the endpoint of the titration by changing colour. Now begin the titration. Acid-base titration is a method often used to determine the concentration of a basic solution given the concentration of an acidic solution is known and vice versa.
Perform an Acid Base Titration conniebisesi 1 of 2. This experiment is to standardize the acid which is to identify its actual concentration. ACID-BASE TITRATION Sample Data Collection and Results Pages Volume of NaOH used in the Titration.
How to write a plan and design experiment Feb. Andrew Dickson of Scripps Institution of Oceanography. Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the.
Rough trial Trial 3 Trial 2 Trial 1 Initial reading mL Z10SML oO0mL O00ML o00ML Final reading mL 4315 mL 21oom L Z145ML Z150mL Volume dispensed mL 2155ML 21Lo5mL 210OmL 21SOML Average volume of NAOH used Trials 1-3. Volume data from the titration of unknown monoprotic acid using standardized. Shao and Agblevor 2015.
To conduct the experiment collect the conical flask HCl methyl orange and glass jar. According to ASTM D1655 2004 the acid number of jet fuels should be less than 01 mg KOHg. The pH changes dramatically with addition of more acid or base.
Trial 1 Trial 2 Trial 3 Initial volume mL 1660 060 1640 Final volume mL 3230 1640 3218 Volume added end-point mL VNaOH 1570 1580 1578 Table 2. The indicator is usually an organic dye that behaves as a weak acid or a weak base. Dissolve exactly 26gm of sodium carbonate in distilled water and transfer all the solution in a long flask.
The Appendix to this report lists some typographical errors found in the references for this report as well as in other papers on this topic. And closing the window. William Piumbroeck Chem 214 Acid-Base Titration Determination of Carbonate and Bicarbonate in a water sample Introduction The purpose of this lab is to determine the concentration of two bases carbonate and bicarbonate by using a potentiometric titration.
Find the concentration of NaOH in molarity M. In addition to the sample an appropriate indicator is added to the titration chamber reflecting the pH range of the equivalence point. Some teachers report yellow with sodium hydroxide purple.
Significant help advice and clarification on all aspects of the CO 2 system in seawater were supplied by Dr. Sodium Carbonate in the other hand is a base. From the titration curve one can determine total number of ionisable groups pK values of different ionisable groups net charge and buffering action of amino.
Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution. An acid-base indicator gives a visual indication of the acidity or basicity of a solution. Briefly a Mettler Toledo T50.
Because the pH of a neutral solution is 7 an indicator that changes color near this pH should be used for an acid-base titration. 5 write the experiment report according to the primary. In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant.
A Thermo Scientific Accucore C18 column 50 mm 21 mm 26 μm was used for the separation with a flow rate of 200 μl min 1 with solvent A 01 vv formic acid in Milli-Q water and. Place a white sheet of paper under Erlenmeyer flask 1 to facilitate the detection of the end point when noting the color change of the indicator. 2 A solution of NaOH was standardized by titration of a known quantity of the primary standard potassium hydrogen phthalate KHP FM 204221 gmol.
A base is a solution that reacts with acids to form salts. They may be weak acids that dissociate and change colour in alkaline solutions. Titration experiment report rollaamalia.
The purpose of this experiment is to observe the titration of hydrochloric acid a strong acid with sodium hydroxide a strong base and acetic acid a weak acid with sodium hydroxide a strong base. When a strong base for example sodiumshow more content Analysis and Calculations The experiment is a strong acid-strong base titration. 23 2013 42 likes.
Fuel corrosion problems are associated with the presence of acids. The method used at WSU to measure acid number is described elsewhere Christensen et al. Hydrochloric acid is a monoprotic acid.
This color change is termed the endpoint of the titration. An acid dissolves in water to show acidic properties which are H ions. It is a sensitive indicator for titration of weak organic bases and ammonia.
Acid base titration 1 Student. Titration of the unknown The titration results using standardized NaOH solution are listed in Table 2. This experiment uses the titration method.
Experiment 565 Prepare methyl red acid-base indicator 1. Titration of 0824 g of potassium hydrogen phthalate required 38314 mL of NaOH to reach the endpoint detected by phenolphthalein. The electrophoresis mobility shift assay EMSA is a rapid and sensitive method to detect proteinnucleic acid interactions 123456It is based on the observation that the electrophoretic.
On the report sheet record the initial reading of the NaOH solution in the buret to the nearest 002 ml. At the endpoint the amounts of strong acid eg H and strong base eg OH are equal. Add some amount of HCl into the titration rod and place a conical flask containing the solution with methyl orange.
Ask your instructor to check your reading and initial your report. Amino acid shows acid-base character. Experiment 1011Part 1 Acid Base Titration.
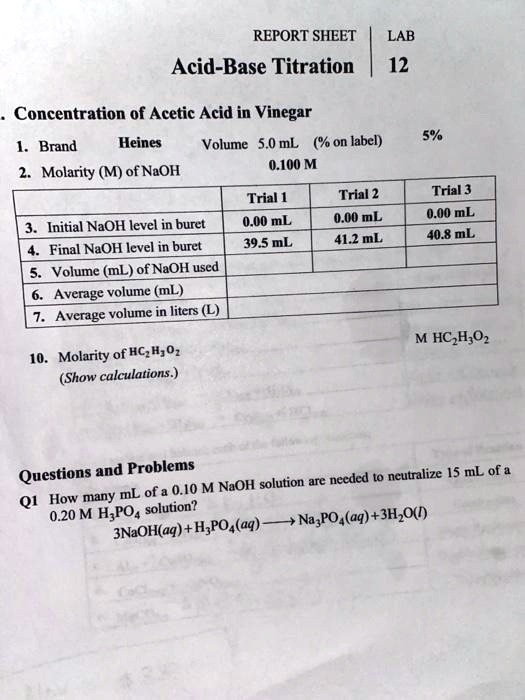
Solved Report Sheet Acid Base Titration Lab 12 Concentration Of Acetic Acid In Vinegar Brand Heines Volume 5 0 Ml On Label Molarity M Of Naoh 0 100 M Trial Trial 2 Initial

Acid Base Titration Lab Report Great College Essay

Acid Base Titration Lab Report The Purpose Of This Experiment Is To Determine The Concentration Of A Solution Of Sodium Hydroxide By Titration Against A Standard Solution Of Potassium Hydrogenphtalate International

0 Comments